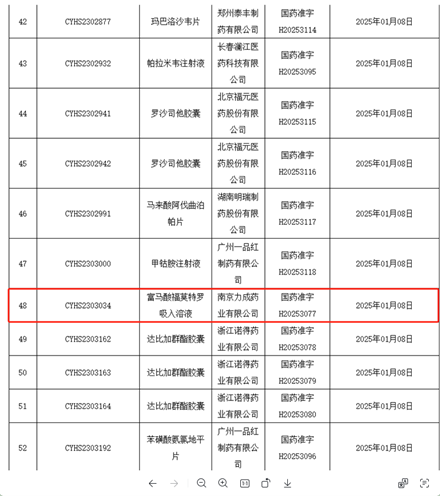
On January 8, 2025, Leecan Pharmaceutical Co., Ltd. obtained production approval for fumarate formoterol inhalation solution, with drug approval number H20253077, becoming the fifth inhalation preparation product approved by Leecan Pharmaceutical Co., Ltd. This product is independently developed, produced, and sold by Leecan Pharmaceutical, and is the first "cold chain product" approved for market by Leecan Pharmaceutical. It further expands and enriches Leecan Pharmaceutical's product pipeline, providing another new option for inhalation medication to patients.
Fumarate formoterol inhalation solution is a long-acting selective β 2-adrenergic receptor agonist that acts locally in the lungs as a bronchodilator after inhalation. It has the effects of relaxing bronchial smooth muscle, relieving bronchial smooth muscle spasm, and anti allergic reactions. This product is used for the maintenance treatment of bronchial stenosis in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema, and can be used for a long time.
Leecan Pharmaceutical adheres to the beautiful original intention of "serving the country with technology and giving back to society" in its entrepreneurship, and is committed to focusing on the field of high-end complex and innovative preparations, breaking monopolies and overcoming barriers through technological innovation, promoting the rapid production and market launch of products, and benefiting Chinese patients with high-quality drugs of national independent brands.
Leecan Pharmaceutical will strictly follow the requirements of the registered and approved process and management regulations for the commercial production of this product, and implement the strictest quality control for the entire production process and every detail, continuously and stably ensuring the quality of each batch of marketed products, helping patients reduce the burden of medication, and helping the country reduce medical costs!


