On December 25, 2024, Leecan obtained production approval for the injection of bendamustine hydrochloride (100 mg and 25 mg), with drug approval numbers H20249778 and H20249779, becoming the first injection product approved by Leecan, and also the first anti-tumor drug of Leecan.
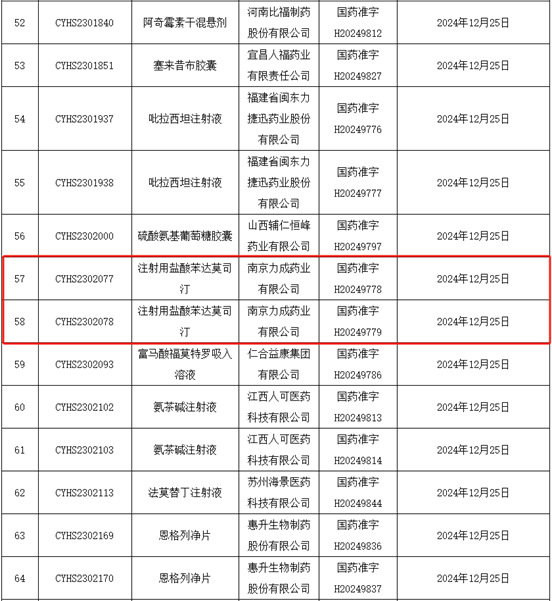
Injection of bendamustine hydrochloride is mainly used clinically to treat chronic lymphocytic leukemia (CLL), as well as indolent B-cell non-Hodgkin lymphoma (NHL) that progresses during or after treatment with rituximab or rituximab containing regimens. The successful approval of this product has greatly expanded Leecan's product pipeline and the therapeutic field of Leecan's products. It will provide new medication options for related cancer patients, making every minute of their lives more dignified and loving!
Leecan will continue to uphold the noble mission of "improving the quality of life through technology", and focus on drugs for respiratory diseases, mental illnesses, immune system diseases, and common chronic diseases. Driven by technological breakthroughs and innovation, we will independently master core technologies, meet people's medication needs with high-quality drugs, and improve patients' quality of life!


