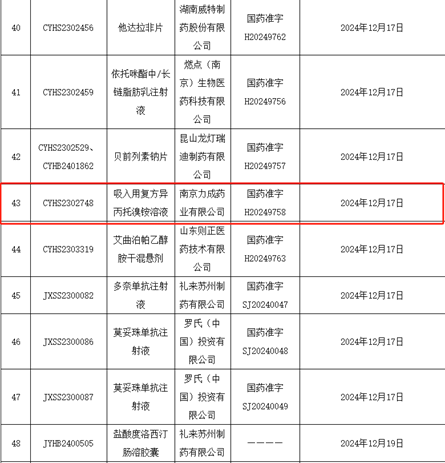
On December 17, 2024, Leecan obtained production approval for its inhalation compound ipratropium bromide solution, with drug approval number H20249758 and tradename Baichang Beiqing. It became the first inhalation compound preparation product and the fourth inhalation preparation product approved by Leecan Pharmaceutical. This product is independently developed, produced, and sold by Leecan Pharmaceutical, and will provide another new option for inhalation medication to patients.
Compound ipratropium bromide solution is a commonly used inhaled medication, which is suitable for patients who require a combination of multiple bronchodilators. It is mainly used to treat reversible bronchospasm related to airway obstructive diseases and can be used for chronic obstructive pulmonary disease, chronic bronchitis, asthma, etc.
The successful approval of this product is another important milestone in the development of Leecan. It is another achievement of Leecan's spirit of continuous improvement and pursuit of excellence in the quality concept. It is also the most powerful witness to Leecan's speed and miracle. This achievement and joy belong to all staff of Leecan!
After the product is approved, it will be quickly put into commercial production, launched, sold, and delivered to patients as quickly as possible. Leecan Pharmaceutical will strictly follow the requirements of the registered and approved process and management regulations, implement the strictest control over every step of the production and inspection process, continuously and stably ensure the quality of each batch of products, and benefit the vast number of patients with high-quality drugs that "reassure ourselves, patients, regulators, and society"!


